
Since 2007
WaterTech
Asiantech Enterprise
Asiantech Enterprise
New Address: 19/140 Sukhunvit Suite Building, Soi Suhkumvit 13 (Saengchan), Sukhunvit Road, Klongtoey-nua, Wattana, Bangkok
ETHO-PROPOX REACTORS: THE STATE OF THE ART
By MM
Polyethoxylation and polypropoxylation reactions are performed, in industry, for preparing non ionic surfactants and polymers. These reactions are highly exothermic (~20 Kcal/mole) and require an efficient heat exchange to avoid the hazard of runaway that is particularly dangerous for the possible intervention, at high temperature, of explosive side reactions. The reaction is, normally, promoted by alkaline catalysts, such as, NaOH or KOH. It has been generally accepted that ethoxylation and propoxylation promoted by alkaline catalysts occur through a nucleophilic substitution SN2. Therefore, the nucleophilicity of the anion, that is a peculiarity of the type of used starter, is very important in promoting the oxirane ring opening. As a consequence, the differences observed in the activities of respectively alkoxide, phenoxide and carboxylic anions are dramatic. The acidity of the starter, is important, too, strongly influencing the proton transfer equilibria and, hence, affecting the oligomers distribution. The alkaline catalysts act as an ionic couple and larger is the ionic radius of the cation more active is the catalyst. On the basis of the above mentioned experimental observations we can write:
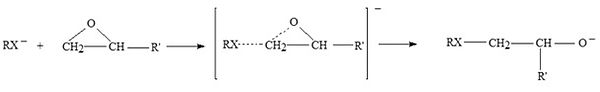
Key factors in alkoxylation technology are respectively: 1) The type of starter employed (hydrophobic like nonylphenol , fatty alcohol, fatty acid or hydrophilic as ethylene or propylene glycols; 2) The solubility of ethylene or propylene oxide in the starter; 3) The types of reactors employed and their performances; 4) The role of kinetics and mass transfer in the process; 5) The safety problems.
All these aspects have been studied and much information can be found in our literature.
The study of the performances of Narrow Range Catalysts is also worth to be mentioned. The reaction is, normally, performed into gas-liquid mixed reactors SBR, Venturi Loop Reactors VLR or Spray Tower Loop Reactors STLR.
The schemes of the three mentioned reactors are respectively below.
As it can be seen, in the first two reactors, the gas phase is dispersed into the liquid one and mass transfer and reaction occur in the same zone of the reactor. On the contrary, in the last reactor, the liquid phase is dispersed in the form of small drops in the gaseous one and mass transfer and reaction occur in two different zones of the reactor.

Ethoxylation and/or propoxylation reaction are carried out in industry in
(a) gas-liquid stirred batch reactor SBR,
(b) venturi loop reactor VLR,
(c) spray tower loop reactor STLR.
In the first two types of reactors SBR and VLR, the gas phase is sparged into the liquid phase; in the third reactor STLR the liquid phase is dispersed into the gaseous phase.
Polyethoxylation and polypropoxylation are necessary to prepare nonionic surfactants and polymers. The starter (ROH) in the synthesis of a nonionic surfactant could be: a fatty alcohol, an alkylphenol, or a fatty acid, i.e., a hydrophobic molecule containing a polar group with an active hydrogen. In this case, the starter will be reacted with ethylene oxide (EO) so as to insert a hydrophilic head in the molecules:
ROH + nEO -> RO(EO)n H
In some cases, propoxylation is performed before ethoxylation to increase the hydrophobicity of the starter (for example, in the case of hexanol) or after ethoxylation to change the foaming characteristics of the final product. The preferred processes are discontinuous semi-batch processes (gaseous alkylene oxide (AO) continuously fed to the liquid substrate), mainly because of the necessity to perform post-treatment operations, such as catalyst removal or bleaching operations, to achieve the required quality features of the product.
In many productions the reaction, normally promoted by alkaline catalysts, such as NaOH or KOH, are frequently performed in stirred batch reactors, see figure (a). However, the use of this type of reactor gives several problems related to productivity and safety. These problems are related to the difficulty in eliminating mass-transfer and heat-transfer limitations generally associated with conventional stirred-tank alkoxylators and with the presence of rotating mechanical parts in contact with the gaseous phase. These problems are particularly important in the case of ethoxylation, because this reaction is much faster than propoxylation and because the ethylene oxide can decompose in gaseous phases with a strongh exothermic reaction.
The presence of a mass-transfer limitation can reduce the productivity of the reactor. Also, the heat-transfer limitation can limit the reactor’s productivity to below that set by mass transfer if the available heat transfer surface per unit volume in the reactor is not high enough to ensure sufficient heat removal.
These reactions are highly exothermic, ΔH = -92 000 J/(mol of ethylene oxide reacted), and require efficient heat exchange to avoid the hazard of runaway reactions that are particularly dangerous because of the possible intervention, at high temperature, of explosive side reactions. The presence of the stirrer may cause gas mixture ignition (dipole formation between the stirrer and the reactor wall and overheating of the mechanical seal) or, if the mechanical seal is damaged or fails, oxide can leak out of the reactor or the seal flush can leak into the reactor. For the above mentioned reasons, SBR can be considered an obsolete technology.
To avoid the problems above mentioned, two alternative reactors can be employed in the alkoxylation reactions: venturi loop reactors (VLR) and spray tower loop reactors (STLR).
In VLR, the gas phase, as for stirred reactors, is dispersed into the liquid phase; on the contrary, in STLR, the liquid phase is dispersed into the gaseous one. In both reactor types mentioned, there are no rotating metallic devices present and the efficiency of heat transfer is ensured by an external thermal exchanger.
Both VLR and STLR reactors give high productivity compared to SBR.
The VLR have excellent mass-transfer performance with less power requirement in comparison to the case of conventional stirred batch reactors, and STLR have shown, for example, in the production of nonylphenol + 9EO, a diminution from 210 to 60 min of required reaction time with respect to that seen in stirred reactors. Kinetics and mass-transfer models for simulating the performances of well-stirred gas-liquid reactors used in the ethoxylation/propoxylation reactions are reported in the literature.
On the contrary, very few paper have been published on the use of spray loop reactors and no detailed papers can be found in the scientific literature about the use of the Venturi loop reactor in the ethoxylation/propoxylation reactions.
In this presentation, a general comparison between the performances of VLR and STLR (best technologies available) in the ethoxylation reaction will be done, studying the maximum productivities of the two reactors in safe conditions.
In the VLR, the pumped liquid passes through a nozzle that provides a high velocity (JET) of fluid to create suction of the gas. In a mixing tube, the high velocity jet attaches itself to the mixing tube wall, resulting in a rapid dissipation of kinetic energy, which creates an intensive mixing with the production of a fine dispersion of gas bubbles. The two-phase mixture that “jets” into the reaction autoclave here also causes intensive mixing. Extensive studies have been reported in the literature that can be useful for a correct design of VLR. The ejector of VLR in many cases can be considered as a device for the complete saturation of the liquid with gas. This occurs because the concentration variation for the reactions is insignificant due to a short contact time. The holding vessel can be considered as a well-stirred reactor.
The productivity of an industrial reactor depends on the following operating conditions: temperature, catalyst concentration, EO feed, and the fixed maximum total pressure of the reactor.
The correct choice of the last two operating parameters is also important for safety. The total pressure also depends on the presence of an inert gas (nitrogen). Nitrogen is introduced into the reactor before ethylene oxide to remove oxygen (the combustible range of EO-air mixtures is between 2,6 and 100%, and to prevent EO decomposition that can also happen suddenly in the absence of air. To prevent EO decomposition, the gas phase in the reactor must be rendered inert with a sufficient amount of nitrogen. Moreover, at the normally used reaction temperature, to limit the formation of other ethylene oxide byproducts which adversely affect the use properties, the inert gas pressure has to be at least 80% of the alkylene oxide partial pressure, a molar gaseous fraction of EO <0.50. The limit becomes lower if hot spots, caused by local catalytic deposits or mechanical friction, could be present, as in the case of an agitated reactor. Many more details on safety conditions (also in the presence of possible EO decomposition flames entering the reactor via a feed line terminating in the vapour space) are reported by Britton. To reach the safety conditions, an initial nitrogen pressure in the range 1-7 bar is generally used.
During syntheses, over time, the volume increases linearly with EO consumption. The total pressure increases until the EO feed is operative then rapidly decreases. The final pressure is higher than the initial one because of the diminution of the volume of the gaseous phase and the increase of the total moles of inert gas in the reactor. The gas molar fraction of EO reaches a maximum and then decreases as a consequence of the inert accumulation in the reactor. The productivity of the system can obviously be increased by increasing the EO feed rate. This increase has a limit that is due to the maximum pressure that can be reached in the reactor.
The venturi loop reactor has some drawbacks that are related to the necessity to have the geometrical parameters of the venturi-type ejector within defined limits.
First of all, when a reactor tower with a large diameter is needed (>1.5-3 m), since ejectors can be viewed as space concentrated distributors, more than one jet may be used to obtain satisfactory performances. Moreover, in alkoxylation reactions, as the liquid strongly increases as a consequence of the reaction (up to a 50-fold increase in the production of some products, such as the polyglycols, is possible) and for a reactor with a small diameter (<1.5), a unique Venturi loop device becomes inadequate to handle the variation condition for the liquid level. For this reason, a multijet arrangement, with the jet starting to operate at different liquid levels, should be used for VLR in the alkoxylation reaction. Moreover, with a high increase in liquid volume, the hydrodynamic behaviour of the reactor changes. In fact, in this case, a nonagitated zone at the bottom of the reactor is formed. In this zone, the liquid has a plug-flow behaviour. So the behaviour becomes similar to that of STLR, as we will see below.
In STLR, the sprayed liquid is dispersed in the form of small liquid drops flying into the alkylene oxide gaseous atmosphere. Drops emerging from an efficient spray nozzle resulted as internally well-mixed drops, leading to a very high mass-transfer rate, and if the average flight time of the drops is long enough, these drops are completely saturated at the end of their flight. In all cases when flight times are much shorter than the ethoxylation reation time (fraction of seconds), the extent of the reaction occurring inside the drops can be disregarded. Hence, in these reactors, mass transfer and chemical reaction occur separately in two distinct zones of the reactor: the mass-transfer zone, corresponding to the zone of drops flying across the gaseous atmosphere, and the reaction zone, corresponding to the slowly flowing liquid-phase collected at the bottom of the reactor. the performances of the STLR are quite similar to those of the VLR in terms of the obtainable maximum productivity (F = 1500 kg/h of EO) in the optimal conditions of reaction. To achieve the maximum productivity in STLR, a high recirculation flow rate is necessary. The required power input for a spray tower loop reactor is of the same order of magnitude as that for a very efficient stirred reactor (self-aspirating stirrer, for example) and is, therefore, greater than that for a Venturi loop reactor.
Just one more analyses about cleaning.
Traditional SBR are always very easy to clean, cleaning cycles are always very short and the generation of wastewater very limited. On the other hand, the cleaning of VLR and STLR is always very complex. If engineering design is not properly done, cleaning time may become very difficult and may take days moreover with generation of large quantities of wastewater.
In conclusion, both VLR and STLR, by not having mechanical stirrers, are safer than mechanically agitated reactors. VLR and STLR can give similar maximum productivities in ethoxylation reactions, but STLR require a power input greater than that for VLR. However, the venturi loop reactor has some drawbacks related to the rigid geometrical parameters that have to be satisfied as concerns the dimensions of the reactor, the liquid level (loop pump), and the nozzle length. In the case of large reactors, the behavior of VLR can also become similar to that of STLR in terms of the power input required for liquid recirculation.
Both VLR and STLR need proper design in order to allow easy, efficient and fast cleaning. In general this is not so simple to achieve.








